Bond Analysis#
Analysis of chemical bonding is crucial to understand the reactivity and properties of any compound. ORCA provides various tools to investigate and visualize chemical bonds based on quantum chemical methods.
Localized Orbitals#
While canonical molecular orbitals yield valuable information, they can be difficult to interpret due to their delocalized nature. This is particularly the case for large molecules. A more convenient alternative is localizing the molecular orbitals to identify bonding overlaps in line with chemically established bonding concepts. The most prominent localization techniques are Foster-Boys (FB) and Pipek-Mezey (PM) localizations.
Example 1: Cr-Cr Bonding in Dichromium Tetraacetate Dihydrate#
In this tutorial we will use a Pipek-Mezey localization to study the bonding situation in dichromium tetraacetate dihydrate, a well-known textbook example of a metal-metal quadruple bond. The PM localization on the previously optimized geometry of the complex is envoked by:
!r2SCAN-3c
%LOC
LOCMET PM
END
*XYZFILE 0 1 structure.xyz
In the output file the details of the localization will be printed after
------------------------------------------------------------------------------
ORCA ORBITAL LOCALIZATION
------------------------------------------------------------------------------
and the summary of the localized molecular orbitals (LMOs) is given. Further, the localized orbitals are stored in the basename.loc file.
--------------------------------------------------------------------------------
LOCALIZED MOLECULAR ORBITAL COMPOSITIONS
--------------------------------------------------------------------------------
The Mulliken populations for each LMO on each atom are computed
The LMO`s will be ordered according to atom index and type
(A) Strongly localized MO`s have populations of >=0.950 on one atom
(B) Two center bond orbitals have populations of >=0.850 on two atoms
(C) Other MO`s are considered to be `delocalized`
FOUND - 20 strongly local MO`s
- 48 two center bond MO`s
- 0 significantly delocalized MO`s
Rather strongly localized orbitals:
MO 47: 32O - 0.964883
[...]
MO 28: 0Cr - 1.014036
Bond-like localized orbitals:
MO 95: 35H - 0.340608 and 30O - 0.658898
[...]
MO 66: 12O - 0.869229 and 9Cr - 0.109159
MO 65: 11O - 0.876956 and 9Cr - 0.092742
MO 64: 11O - 0.587896 and 5C - 0.415626
MO 63: 11O - 0.793870 and 5C - 0.182382
MO 62: 10O - 0.869257 and 9Cr - 0.109543
MO 61: 9Cr - 0.488121 and 0Cr - 0.526559
MO 60: 9Cr - 0.472239 and 0Cr - 0.471944
MO 59: 9Cr - 0.541468 and 0Cr - 0.525393
MO 58: 9Cr - 0.541872 and 0Cr - 0.524219
MO 57: 8C - 0.463590 and 7C - 0.609424
[...]
MO 48: 1O - 0.869095 and 0Cr - 0.109446
Localized MO's were stored in: orca.loc
From this output, we can see that ORCA identified 4 "bond-like" LMOs between the two chromium atoms with
almost 50/50 electron populations. This indicates four relevant bonding contributions in line with
the expected σ-, 2×π-, and δ-bonds. To visualize these bonds after successful calculation, we can utilize orca_plot. To do so, we rename the basename.loc file to loc.gbw and
call orca_plot in its interactive mode (-i).
orca_plot loc.gbw -i
We can now plot the LMOs like normal MOs by choosing the respective orbital number via option 2 - Enter no of orbital to plot (for high-quality plots, we adjusted the number of grid intervals to 100 via 4 - Enter number of grid intervals). The obtained .cube files can now be visualized with ChimeraX or any other program of choice.
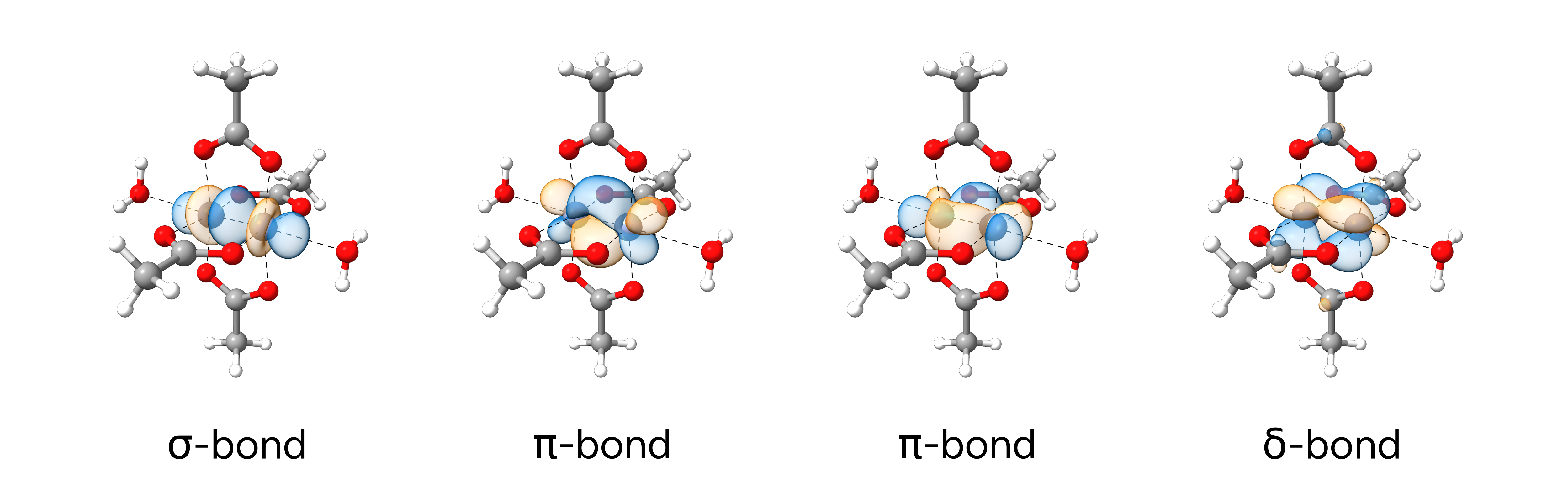
Figure: Localized Molecular Orbitals of the Cr-Cr quadruple bond.#
We see, that the visualized LMOs perfectly match our expectations.
Structures#
Dichromium Tetraacetate Dihydrate
36
Cr 3.63292803675582 4.78285607739625 -0.63639358256050
O 2.34785749491859 4.48652278178970 -2.12009832625825
O 5.13493229672345 5.30466992720158 0.58344087799744
O 4.73076207322555 3.26794262826257 -1.29923843647248
O 2.73716250606921 6.52977972635174 -0.24223293301648
C 1.34714372264977 3.77755038643592 -1.79342034446331
C 0.28882874973655 3.46634459556225 -2.81104036856054
C 4.49276620679543 2.16989237310360 -0.70883979137882
C 5.25046716873396 0.93386548365656 -1.09583669544289
Cr 2.72281835907000 3.84276439529425 0.61069113865845
O 4.00842988963071 4.13941356801647 2.09374138676694
O 1.22096071474473 3.32092019482001 -0.61027879254538
O 1.62460073626451 5.35790230455260 1.27270500588077
O 3.61876789645852 2.09613885710674 0.21605424661246
C 5.00922985477937 4.84802707447584 1.76669231158828
C 6.06814229046835 5.15890455396898 2.78377912360696
C 1.86247448824412 6.45579094664928 0.68192448021066
C 1.10332879431268 7.69141916931006 1.06747861321807
H 0.53643199444775 3.91479455350269 -3.77316600096031
H 0.19363516930407 2.38162440858367 -2.91892115129840
H -0.67646851589299 3.84412291439512 -2.46031116829040
H 5.82036413122407 0.57284519899174 -0.23393409530863
H 4.54651848337104 0.14401323934197 -1.37402597678676
H 7.03354834642659 4.78268981767564 2.43171518212094
H 6.16212212277575 6.24359469909520 2.89312979871985
H 5.82180818584082 4.70895962627934 3.74553075821521
H 5.92625733753088 1.14156035056277 -1.92544044162556
H 0.42730948584067 7.48388311065848 1.89692409507629
H 1.80630411261855 8.48232991810399 1.34513931752921
H 0.53353311936446 8.05099140285292 0.20489050593958
O 1.41030940144609 2.25034914090934 2.03754830017122
H 0.69456507333094 2.16354974421073 1.39521339423714
O 4.95323562321733 6.38396944730196 -2.06427896840346
H 4.37901353165716 7.13631180534098 -1.87274555994989
H 5.66469043982413 6.46222396268224 -1.41594550916286
H 1.98371067809123 1.49606161555666 1.85174960593545