Infrared and Raman¶
The prediction of infrared (IR) and (non-resnonant) Raman spectra are nowadays a straightforward task in computational chemistry. Let us use ORCA to predict the fundamental frequencies and intensities for benzene.
Figure: D6h symmetric structure of benzene.¶
Because of its symmetry, the selection rules predict that these bands are mutually exclusive, with \(A_{2u}\) and \(E_{1u}\) modes being the most IR active (\(B_{1u}, B_{2u}\) and \(E_{2u}\) modes are less intense), while the \(A_{1g}, E_{1g}\) and \(E_{2g}\) are Raman active. For comparison, the complete set of experimental values can be found on NIST's website.
Predicting infrared spectra¶
The optimization of the molecular structure and subsequent calculation of the vibrational
frequencies, e.g. with DFT can be envoked via simple keywords OPT and FREQ:
!BP86 DEF2-SVP OPT FREQ
*XYZFILE 0 1 structure.xyz
For Grimme's efficient r2SCAN-3c composite DFT method the respective input is:
!r2SCAN-3c OPT FREQ
If no analytical Hessian is available for the chosen method, the NUMFREQ keyword can be used to request a numerical Hessian calculation.
After the successful calculation, the vibrational frequencies will be printed:
-----------------------
VIBRATIONAL FREQUENCIES
-----------------------
Scaling factor for frequencies = 1.000000000 (already applied!)
0: 0.00 cm**-1
1: 0.00 cm**-1
2: 0.00 cm**-1
3: 0.00 cm**-1
4: 0.00 cm**-1
5: 0.00 cm**-1
6: 405.17 cm**-1
7: 405.28 cm**-1
8: 626.34 cm**-1
9: 626.55 cm**-1
[...]
followed by the vibrational modes and the IR spectrum.
-----------
IR SPECTRUM
-----------
Mode freq eps Int T**2 TX TY TZ
cm**-1 L/(mol*cm) km/mol a.u.
----------------------------------------------------------------------------
6: 405.17 0.000000 0.00 0.000000 ( 0.000000 0.000000 -0.000000)
7: 405.28 0.000000 0.00 0.000000 ( 0.000000 -0.000000 0.000109)
8: 626.34 0.000000 0.00 0.000000 ( 0.000000 0.000000 0.000000)
9: 626.55 0.000000 0.00 0.000000 (-0.000000 0.000000 -0.000000)
10: 676.63 0.025862 130.70 0.011928 (-0.000000 0.000000 -0.109214)
[...]
where the dipole derivatives necessary to compute the IR intensities are printed after each frequency.
Plotting the IR spectrum¶
The IR spectrum can then be immediately generated with any suitable GUI
like Avogadro 2. Just open the saved output file basename.out and click on Analysis → Plot Spectra... to open a new window were the IR spectrum will be plotted:
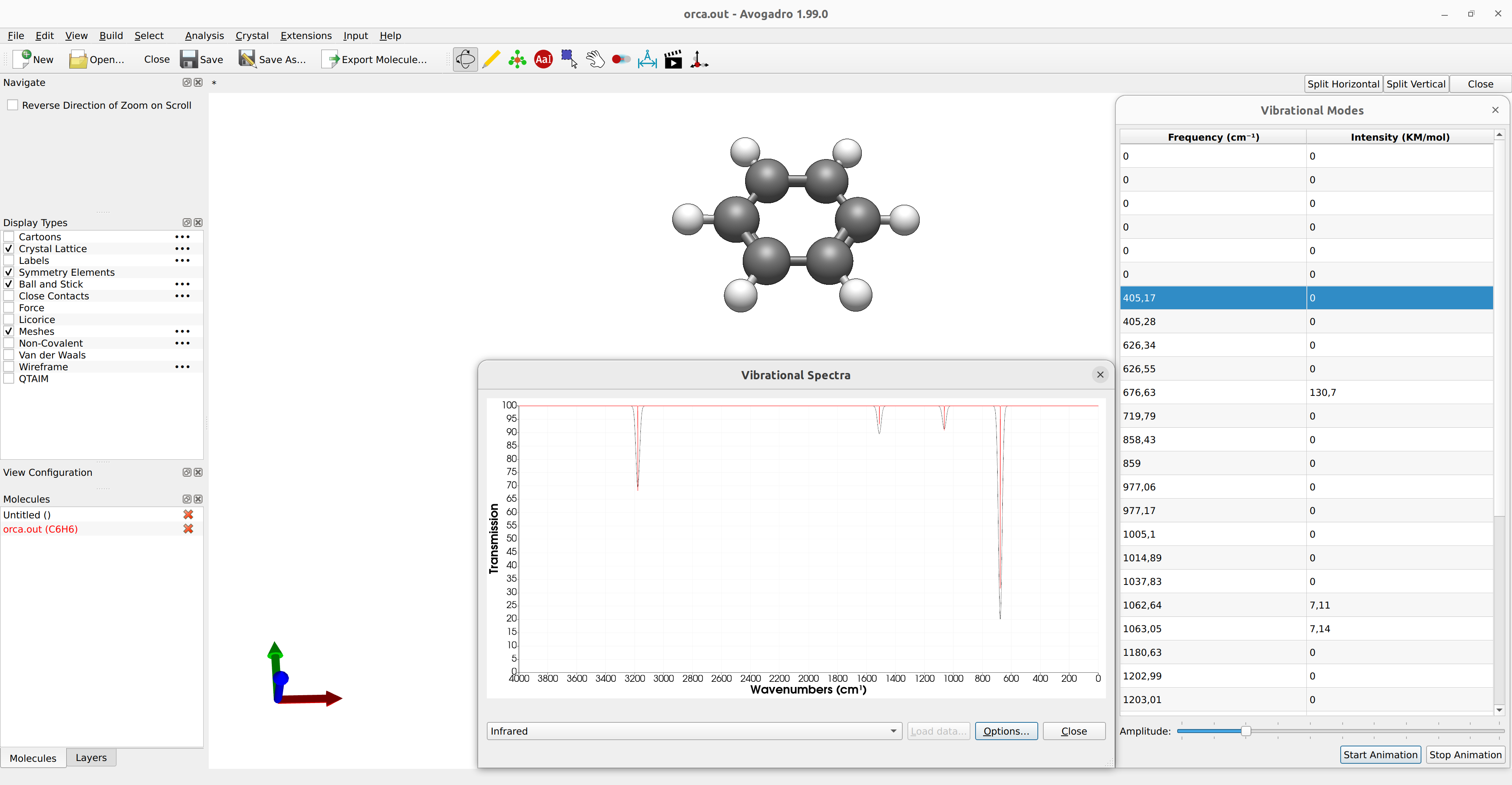
Figure: IR spectrum plotted with Avogadro 2.¶
Visualizing modes¶
In order to have a better assignment of the IR peaks, one can click on the corresponding frequency in the main Avogadro window and then on Start Animation to make a small movie of the mode displacement.
As an example, if one chooses the \(A_{2u}\) IR active mode at \(668 cm^{-1}\), that in textbooks is commonly assigned as an out-of-plane C-H bending, one gets:
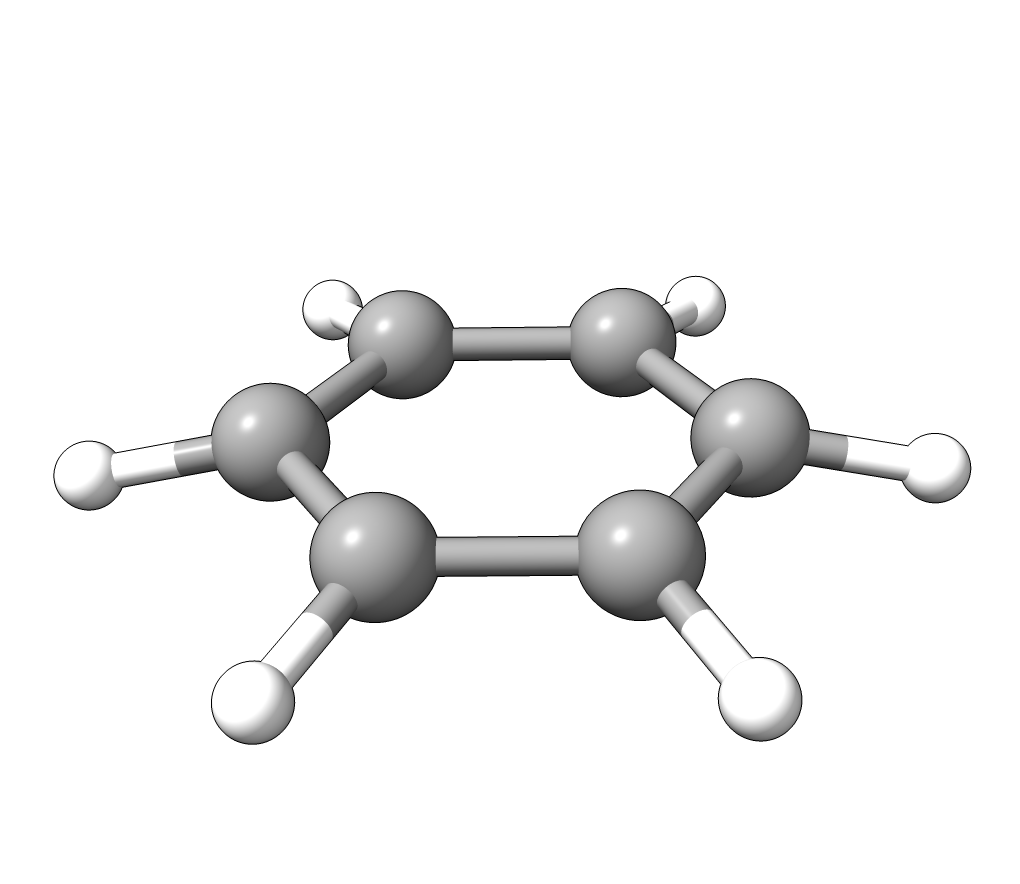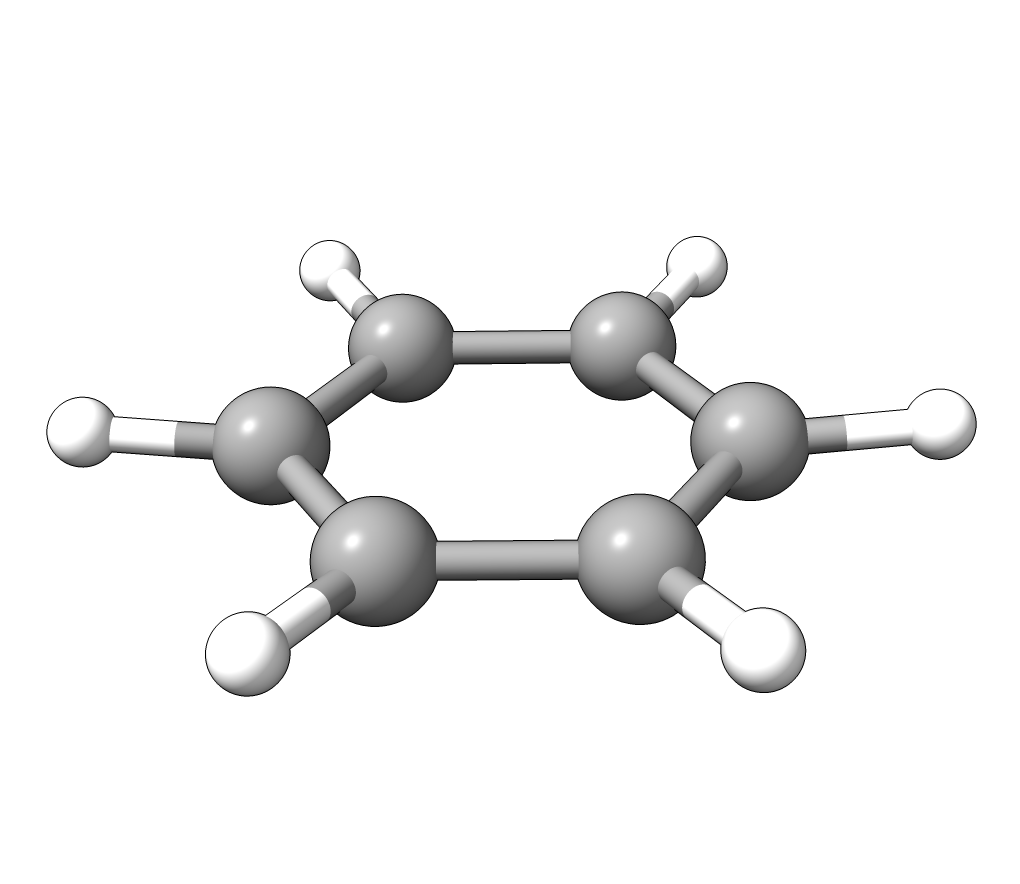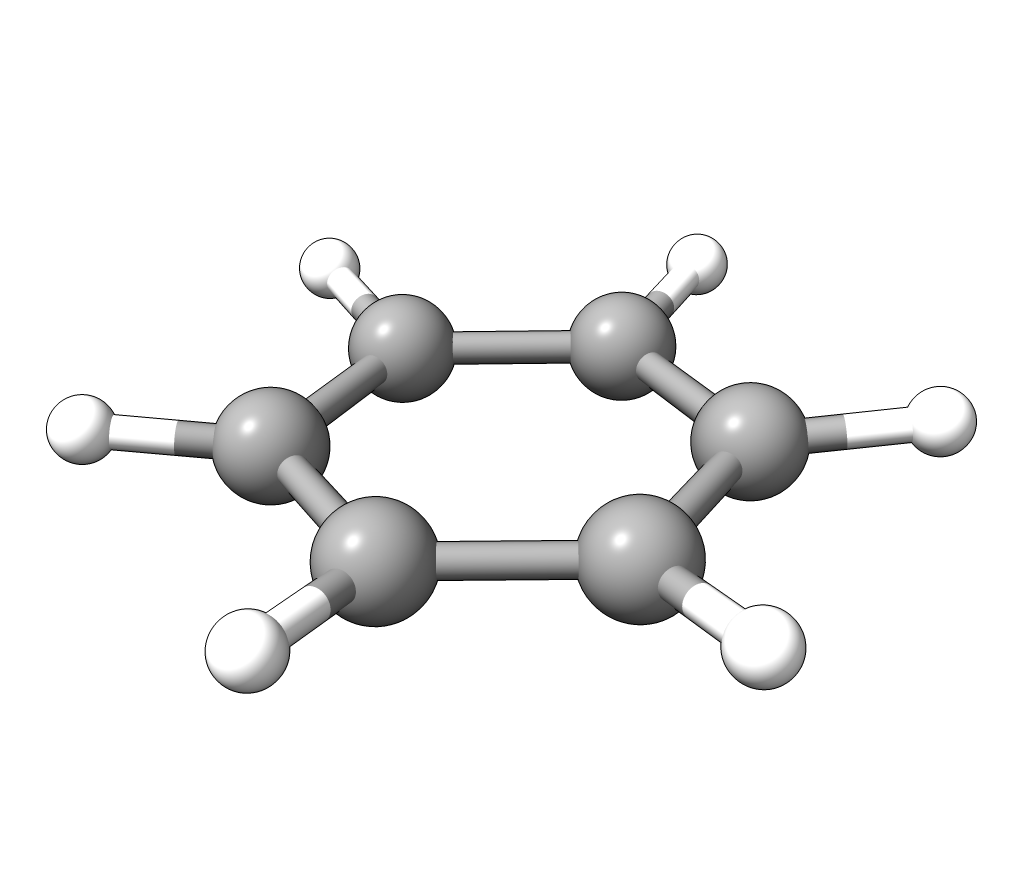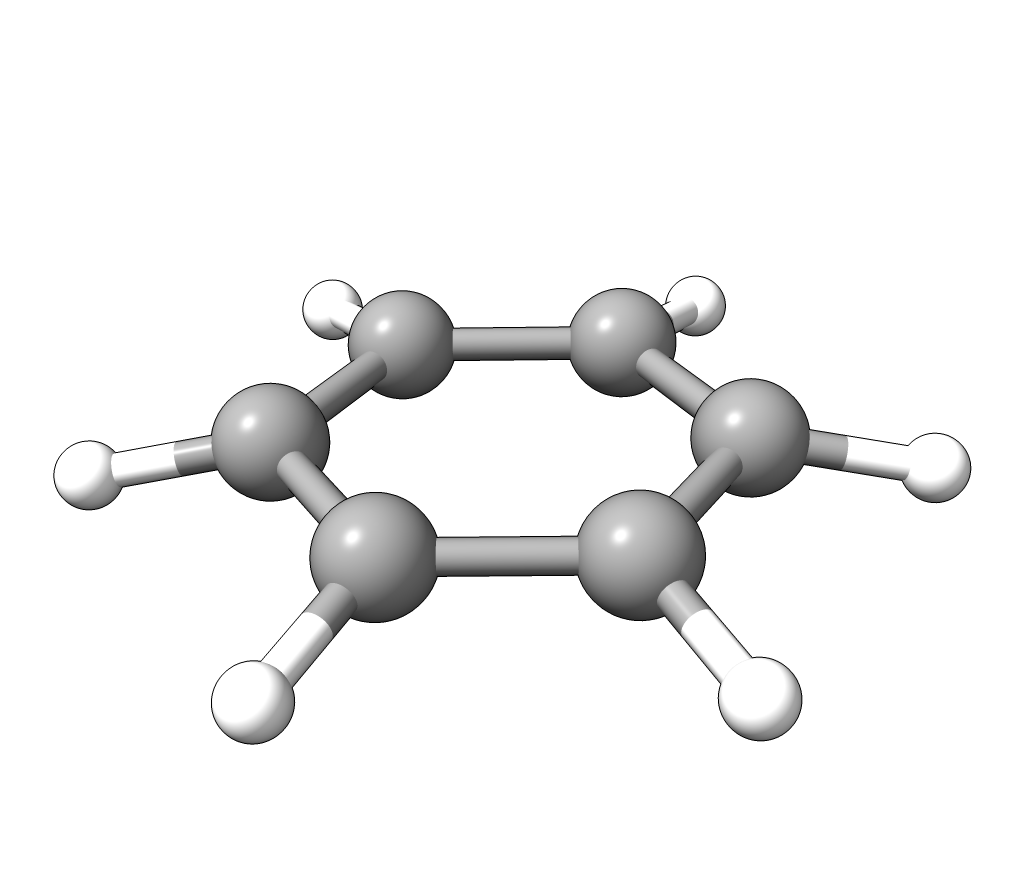
Animation: \(A_{2u}\) IR active mode of benzene.¶
exactly as expected. The symmetry breaking creates a temporary dipole one the benzene molecule, and that allows for intensity on a IR transition.
Note
The line broadening must be included ad hoc here because it is related to the lifetimes of vibrational excited states and structural parameters that were not accounted for in the calculation and might vary depending on the experimental conditions.
Important
Be aware that we are predicting only fundamental transitions, so no overtones and combination bands are included!
Here is a table comparing the calculated and experimental values for the IR fundamentals:
Mode |
Experiment / cm-1 |
Predicted / cm-1 |
|---|---|---|
\(E_{2u}\) Ring deform |
410 |
405 |
\(A_{2u}\) C-H bend |
673 |
676 |
\(B_{1u}\) Ring deform |
1010 |
1005 |
\(E_{1u}\) C-H bend |
1038 |
1037 |
\(B_{2u}\) C-H bend |
1150 |
1180 |
\(E_{1u}\) C=C str |
1486 |
1511 |
\(B_{1u}\) C-H str |
3068 |
3179 |
\(E_{1u}\) C-H str |
3063 |
3116 |
Predicting Raman spectra¶
In a similar way, the non-resonant Raman spectra composed of fundamental transitions can be predicted. The main difference here is that the derivatives of the polarizability have to be computed numerically, so that the !NUMFREQ flag has to be used, together with a keyword under %ELPROP to request the calculation of polarizabilties:
!r2SCAN-3c OPT NUMFREQ
%ELPROP
POLAR 1
END
*XYZFILE 0 1 structure.xyz
Note
Analytical Hessians are not available for Raman spectra calculations. Therefore, NUMFREQ must be used.
Here POLAR 1 means that the polarizability is computed analytically, by solving the CP-SCF equations. However, this option is only available for regular DFT and HF. Please check the ORCA manual for all available options.
After successful termination the Raman spectrum is printed after the IR section and the Raman spectrum can be plotted with Avogadro analogous to the IR spectrum.
--------------
RAMAN SPECTRUM
--------------
Mode freq (cm**-1) Activity Depolarization
-------------------------------------------------------------------
6: 406.22 0.000000 0.066146
7: 406.38 0.000001 0.035663
8: 620.29 6.321364 0.748913
9: 626.43 6.122879 0.750000
10: 677.12 0.000007 0.078694
11: 720.92 0.002478 0.749995
12: 859.04 3.481939 0.749999
[...]
It is important to say the Raman intensities are a more complicated subject than those of IR and are much more dependent on the experimental setup, that is why we print here the so-called "Raman Activity" instead [Hess2002], because it is setup invariant.
Again, we can compile a table comparing the calculated and experimental values for the Raman fundamentals:
Mode |
Experiment / cm-1 |
Predicted / cm-1 |
|---|---|---|
\(E_{2g}\) Ring deform |
606 |
620 |
\(E_{1g}\) C-H bend |
849 |
859 |
\(A_{1g}\) Ring str |
992 |
977 |
\(E_{2g}\) C-H bend |
1178 |
1197 |
\(A_{2g}\) C-H bend |
1326 |
1374 |
\(E_{2g}\) Ring str |
1606/1585 |
1630 |
\(E_{2g}\) C-H str |
3047 |
3177 |
\(A_{1g}\) C-H str |
3062 |
3186 |
Scaling frequencies¶
Please also note the Scaling factor for frequencies printed in the output just before the frequency
values. These are empirical factors that can be used to multiply all frequencies and correct for
errors from theory [Martin2014]. It can be controlled by setting the SCALFREQ flag under %FREQ. For example, the previous reference suggest a scaling factor of 0.9956 for BP86/DEF2-SVP.
!BP86 DEF2-SVP FREQ
%FREQ
SCALFREQ 0.9956
END
* XYZFILE 0 1 structure.xyz
Note
If one is using Avogadro, these scaling factor can be added later by setting the "Scale Factor" on the "Infrared Spectra Settings" tab shown before.
Warning
Negative frequencies mean that your system is not in real minimum! Please check the Removing negative frequencies section for more info on that.
Starting structures¶
Benzene (D6h)
12
H -1.242909 -2.152782 0.000000
C -0.695566 -1.204756 0.000000
C 0.695566 -1.204756 0.000000
H 1.242909 -2.152782 0.000000
C 1.391133 0.000000 0.000000
H 2.485819 0.000000 0.000000
C 0.695566 1.204756 0.000000
H 1.242909 2.152782 0.000000
C -0.695566 1.204756 0.000000
H -1.242909 2.152782 0.000000
C -1.391133 0.000000 0.000000
H -2.485819 0.000000 0.000000