Thermodynamics#
If you have successfully done a Vibrational frequencies calculation, now you have access to the thermodynamic functions such as enthalpy (\(H\)), entropy (\(S\)) and the Gibbs free energy (\(G\))!
Let's follow an example and use this to predict the relative enthalpy ordering of some butadiene isomers.
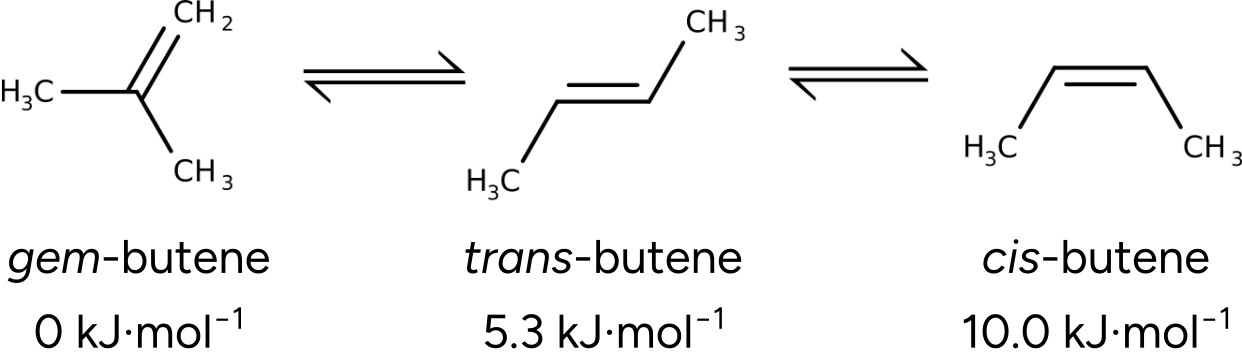
Figure: Experimental relative free energies of gem-, trans-, and cis-butene (T = 298.15 K, p = 1 atm).#
using the following keywords and the geminal isomer as an example:
!B3LYP D4 DEF2-TZVP OPT FREQ
* XYZFILE 0 1 gem-butene.xyz
Thermodynamic functions#
Assuming you had !FREQ on the input, immediately after the frequencies and normal modes, one will see that the thermochemistry section starts:
Temperature ... 298.15 K
Pressure ... 1.00 atm
Total Mass ... 56.11 AMU
Quasi RRHO ... True
Cut-Off Frequency ... 1.00 cm^-1
Note
These properties will be computed for a given temperature and pressure, assuming a gas-phase molecule and using statistical mechanics. For thermodynamics in solution, check the Implicit Solvation Models section.
Inner energy#
The first function is the inner energy (\(U\)), that is computed following the output:
------------
INNER ENERGY
------------
The inner energy is: U= E(el) + E(ZPE) + E(vib) + E(rot) + E(trans)
E(el) - is the total energy from the electronic structure calculation
= E(kin-el) + E(nuc-el) + E(el-el) + E(nuc-nuc)
E(ZPE) - the the zero temperature vibrational energy from the frequency calculation
E(vib) - the the finite temperature correction to E(ZPE) due to population
of excited vibrational states
E(rot) - is the rotational thermal energy
E(trans)- is the translational thermal energy
Summary of contributions to the inner energy U:
Electronic energy ... -157.18614555 Eh
Zero point energy ... 0.10746477 Eh 67.44 kcal/mol
Thermal vibrational correction ... 0.00246995 Eh 1.55 kcal/mol
Thermal rotational correction ... 0.00141627 Eh 0.89 kcal/mol
Thermal translational correction ... 0.00141627 Eh 0.89 kcal/mol
-----------------------------------------------------------------------
Total thermal energy -157.07337829 Eh
The Electronic energy is what you get from the FINAL SINGLE POINT ENERGY, then the Zero-Point Energy (ZPE) is calculated, together with the Thermal correction, which has to do with the rotational, vibrational and translational degrees of freedom.
The Total correction is amount the of energy you have to add to your FINAL SINGLE POINT electronic energy to convert \(E_{el}\) into \(U\).
Enthalpy#
Then the calculation of the enthalpy. That is simply \(U + k_BT\):
--------
ENTHALPY
--------
The enthalpy is H = U + kB*T
kB is Boltzmann's constant
Total thermal energy ... -157.07337829 Eh
Thermal Enthalpy correction ... 0.00094421 Eh 0.59 kcal/mol
-----------------------------------------------------------------------
Total Enthalpy ... -157.07243408 Eh
Again, the Thermal Enthalpy correction is what one has to add to \(U\) to compute \(H\).
Entropy#
The entropy term follows:
-------
ENTROPY
-------
The entropy contributions are T*S = T*(S(el)+S(vib)+S(rot)+S(trans))
S(el) - electronic entropy
S(vib) - vibrational entropy
S(rot) - rotational entropy
S(trans)- translational entropy
The entropies will be listed as multiplied by the temperature to get
units of energy
Electronic entropy ... 0.00000000 Eh 0.00 kcal/mol
Vibrational entropy ... 0.00393432 Eh 2.47 kcal/mol
Rotational entropy ... 0.01154964 Eh 7.25 kcal/mol
Translational entropy ... 0.01805279 Eh 11.33 kcal/mol
-----------------------------------------------------------------------
Final entropy term ... 0.03353675 Eh 21.04 kcal/mol
with its different components and a Final entropy term that is already multiplied by the temperature, the \(TS\) term that is latter used for the free energy.
Gibbs free energy#
Finally, the free energy term is printed:
-------------------
GIBBS FREE ENERGY
-------------------
The Gibbs free energy is G = H - T*S
Total enthalpy ... -157.07243408 Eh
Total entropy correction ... -0.03353675 Eh -21.04 kcal/mol
-----------------------------------------------------------------------
Final Gibbs free energy ... -157.10597083 Eh
For completeness - the Gibbs free energy minus the electronic energy
G-E(el) ... 0.08017472 Eh 50.31 kcal/mol
The last term, G-E(el) is the term that you would have to add to your electronic energy to transform \(E_{el}\) into \(G^o\) at a given temperature and pressure.
Note
This G-E(el) only depends on the geometry and frequencies, and thus can be computed in a lower level method, such as DFT, and later combined with the \(E_{el}\) of a higher level method, such as DLPNO-CCSD(T) to achieve a very good prediction of the \(G^o\)!
Comparison to experiment#
The Final Gibbs free energy values yield relative free energies of \(3.4\) \(kJ mol^{-1}\) and \(7.1\) \(kJ mol^{-1}\).
To improve the agreement with the experimental data, the electronic energy component \(E_{el}\) can also
be computed at the DLPNO-CCSD(T) level. The respective values are given by the FINAL SINGLE POINT ENERGY.
!DLPNO-CCSD(T) aug-cc-pVTZ AUTOAUX RIJCOSX
* XYZFILE 0 1 butene_optimized_dft.xyz
These electronic energies are now corrected by the G-E(el) values from the DFT calculations.
Compound |
\(\Delta E_{DFT} + \Delta (G-E_{el, DFT})\) |
\(\Delta E_{CCSD(T)} + \Delta (G-E_{el, DFT})\) |
\(\Delta G_{Exp.}\) |
|---|---|---|---|
gem-butene |
0.0 |
0.0 |
0.0 |
trans-butene |
3.4 |
4.8 |
6.2 |
cis-butene |
7.1 |
8.6 |
7.8 |
Thermodynamics at different temperatures#
To compute these thermodynamic properties at different temperatures, or at several, one can use:
%FREQ TEMP 77, 298, 330, 450 END
and the thermodynamic functions will be separated by the headers:
--------------------------
THERMOCHEMISTRY AT 77K
--------------------------
[...]
--------------------------
THERMOCHEMISTRY AT 298K
--------------------------
[...]
and so on.
Changing the temperature after the calculation#
If you want to compute thermodynamic functions after you calculation has already finished, that can be done setting a simple input such as:
!PRINTTHERMOCHEM
%GEOM
INHESSNAME "basename.hess"
END
%FREQ TEMP 290, 295, 300
END
* XYZFILE 0 1 geometry.xyz
as one can save a lot of time from recalculations.
Note
One could use this to also include isotopic effects, by changing the masses in the basename.hess file and recomputing the thermodynamics (see Kinectic Isotope Effects (KIE)).
Structures#
gem-butene
12
C -1.43333641710982 0.99750434133223 -0.11833314354528
C -0.18475482717332 0.62759099420377 -0.39181661341896
C 0.13812904158972 -0.69904137413719 -1.01927936124571
C 0.99530997982103 1.50886801088864 -0.09403775990942
H 0.66526364427023 -0.56158725840381 -1.96888083944946
H -0.75974400764642 -1.28775885666908 -1.20721189335260
H 0.80403526745525 -1.28218576008005 -0.37502483269079
H -2.27650846609433 0.35335064429450 -0.33755880129752
H -1.65451309098319 1.95681047014208 0.33410795230859
H 1.55825458218721 1.72631922619331 -1.00745285372439
H 0.69222808602999 2.45448923561900 0.35550308769672
H 1.69049620765366 1.00988032661660 0.58883505862882
trans-butene
12
C 1.31005769169158 0.51452879141346 0.05804263697398
C -0.04819313785594 -0.09253207982925 0.22254234005303
H 1.79539655204235 0.66757127845972 1.02702463158789
H 1.25730597675553 1.47886263622368 -0.45054128881957
H 1.96882389016151 -0.13975288898310 -0.52148647916325
C -1.18726477431566 0.45327229214244 -0.18976017691771
C -2.54552738268524 -0.15377457291609 -0.02524544056845
H -3.03091939506206 -0.30671024889310 -0.99422100546098
H -3.20425241496413 0.50046308281000 0.55438662370226
H -2.49276412960279 -1.11816170340100 0.48323797563451
H -0.08496173672054 -1.05895215397092 0.72255102983275
H -1.15050113944460 1.41969556694416 -0.68977084685447
cis-butene
12
C 1.47970360709777 -0.47841743730424 0.16222562758688
C 0.02367038098010 -0.80795303233869 0.28562414393012
H 1.66007607442031 0.57369355392531 -0.05439609169518
H 1.94416186497463 -1.06912909934475 -0.63340002033858
H 2.01226039794254 -0.72697631354996 1.08545344375310
C -1.01190540852076 0.02068494444637 0.16549233572241
H -0.19509791907163 -1.85098304967194 0.49893762893694
C -0.98084493396470 1.49003154320377 -0.12391775381971
H -2.00537265760596 -0.40248869126449 0.28901974823120
H -1.46501764033373 2.05312506426319 0.67997591564265
H 0.02985297930524 1.87869926998700 -0.24179411861463
H -1.53582674522382 1.71592324764843 -1.03973085933522